Evolution in Action
Jonathan Weiner
Reprinted with Permission
Weiner, J. Evolution in Action. Natural History: 47-51, November, 2005
Charles Darwin’s wife, Emma, was terrified that they would be separated for eternity, because she would go to heaven and he would not. Emma confessed her fears in a letter that Charles kept and treasured, with his reply to her scribbled in the margin: “When I am dead, know that many times, I have kissed and cryed over this.”
Close as they were, the two could hardly bear to talk about Darwin’s view of life. And today, those of us who live in the United States, by many measures the world’s leading scientific nation, find ourselves in a house divided. Half of us accept Darwin’s theory, half of us reject it, and many people are convinced that Darwin burns in hell. I find that old debate particularly strange, because I’ve spent some of the best years of my life as a science writer peering over the shoulders of biologists who actually watch Darwin’s process in action. What they can see casts the whole debate in a new light—or it should.
Darwin himself never tried to watch evolution happen. “It may metaphorically be said,” he wrote in the Origin of Species,
that natural selection is daily and hourly scrutinizing, throughout the world, the slightest variations; rejecting those that are bad, preserving and adding up all that are good; silently and insensible working, when ever and wherever opportunity offers….We see nothing of these slow changes in progress, until the hand of time has marked the lapse of ages.
Darwin was a modest man who thought of himself as a plodder (one of his favorite mottoes was, “It’s dogged as does it”). He thought evolution plodded too. If so, it would be more boring to watch evolution than to watch drying paint. As a result, for several generations after Darwin’s death, almost nobody tried. For most of the twentieth century the only well-known example of evolution in action was the case of peppered moths in industrial England. The moth had its picture in all the textbooks, as a kind of special case.
Darwin's Finches
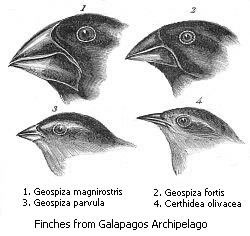 Then, in 1973, a married pair of evolutionary biologists, Peter and Rosemary Grant, now at Princeton University, began a study of Darwin’s process in Darwin’s islands, the Galapagos, watching Darwin’s finches. At first, they assumed that they would have to infer the history of evolution I the islands from the distribution of the various finch species, varieties, and populations across the archipelago. That is pretty much what Darwin had done, in broad strokes, after the Beagle’s five-week survey of the islands in 1835. But the Grants soon discovered that at their main study site, a tiny desert island called Daphne Major, near the center of the archipelago, the finches were evolving rapidly. Conditions on the island swung wildly back and forth from wet years to dry years, and finches on Daphne adapted to each swing, from generation to generation. With the help of a series of graduate students, the Grants began to spend a good part of each year on Daphne, watching evolution in action as it shaped and reshaped the finches’ beaks.
Then, in 1973, a married pair of evolutionary biologists, Peter and Rosemary Grant, now at Princeton University, began a study of Darwin’s process in Darwin’s islands, the Galapagos, watching Darwin’s finches. At first, they assumed that they would have to infer the history of evolution I the islands from the distribution of the various finch species, varieties, and populations across the archipelago. That is pretty much what Darwin had done, in broad strokes, after the Beagle’s five-week survey of the islands in 1835. But the Grants soon discovered that at their main study site, a tiny desert island called Daphne Major, near the center of the archipelago, the finches were evolving rapidly. Conditions on the island swung wildly back and forth from wet years to dry years, and finches on Daphne adapted to each swing, from generation to generation. With the help of a series of graduate students, the Grants began to spend a good part of each year on Daphne, watching evolution in action as it shaped and reshaped the finches’ beaks.
At the same time, a few biologists began making similar discoveries elsewhere in the world. One of them was John A. Endler, an evolutionary biologist at the University of California, Santa Barbara, who studied Trinidadian guppies. In 1986 Endler published a little book called Natural Selection in the Wild, in which he collected and reviewed all of the studies of evolution in action that had been published to date. Dozens of new field projects were in progress. Biologists finally began to realize that Darwin had been too modest. Evolution by natural selection can happen rapidly enough to watch.
Now the field is exploding. More than 250 people around the world are observing and documenting evolution, not only in finches and guppies, but also in aphids, flies, graylings, monkeyflowers, salmon, and sticklebacks. Some workers are even documenting pairs of species—symbiotic insects and plants—that have recently found each other, and observing the pairs as they drift off into their own world together like lovers in a novel by D.H. Lawrence.
The Grants’ own study gets more sophisticated every year. A few years ago, a group of molecular biologists working with the Grants nailed down a gene that plays a key role in shaping the beaks of finches. The gene codes for a signaling molecule called bone morphogenic protein 4 (BMP4). Finches with bigger beaks tend to have more BMP4, and finches with smaller beaks have less. In the laboratory, the biologists demonstrated that they could sculpt the beaks themselves by adding or subtracting BMP4. The same gene that shapes the beak of the finch in the egg also shapes the human face in the womb.
Human Involvement
Some of the most dramatic stories of evolution in action result from the pressures that human beings are imposing on the planet. As Stephen Palumbi, an evolutionary biologist at Stanford University, points out, we are changing the course of evolution for virtually every living species everywhere, with consequences that are sometimes the opposite of what we might have predicted, or desired.
Take trophy hunting. Wild populations of bighorn mountain sheep are carefully managed in North America for hunters who want a chance to shoot a ram with a trophy set of horns. Hunting permits can cost well into the six figures.
On Ram Mountain, in Alberta, Canada, hunters have shot the biggest of the bighorn rams for more than thirty years. And the result? Evolution has made the hunters’ quarry scarce. The runts have had a better chance than the giants of passing on their genes. So on Ram Mountain the rams have gotten smaller, and their horns are proportionately smaller yet.
Or take fishing, which is economically much more consequential. The populations of Atlantic cod that swam for centuries off the coasts of Labrador and Newfoundland began a terrible crash in the late 1980s. In the years leading up to the crash, the cod had been evolving much like the sheep on Ram Mountain. Fish that matured relatively fast and reproduced relatively young had the better chance of passing on their genes; so did the fish that stayed small. So even before the population crashed, the average cod had been shrinking.
We often seem to lose out wherever we fight hardest to control nature. Antibiotics drive the evolution of drug-resistant bacteria at a frightening pace. Sulfonamides were introduced in the 1930s, and resistance to them was first observed a decade later. Penicillin was deployed in 1943, and the first penicillin resistance was observed in 1946. In the same way, pesticides and herbicides create resistant bugs and weeds.
Palumbi estimates that the annual bill for such unintended human-induced evolution runs to more than $100 billion in the U.S. alone. Worldwide, the pressure of global warming, fragmented habitats, heightened levels of carbon dioxide, acid rain, and the other myriad of perturbations people impose on the chemistry and climate of the planet- all change the terms of the struggle for existence in the air, in the water, and on land. Biologists have begun to worry about those perturbations, but global change may be racing ahead of them.
Microevolution
To me, the most interesting news in the global evolution watch concerns what Darwin called “that mystery of mysteries, the origin of species.”
The process whereby a population acquires small, inherited changes through natural selection is known as microevolution. Finches get bigger, fish gets smaller, but a finch is still a finch and a fish is still a fish. For people who reject Darwin’s theory, that’s the end of the story: no matter how many small, inherited changes accumulate, they believe, natural selection can never make a new kind of living thing. The kinds, the species, are eternal.
Darwin argued otherwise. He thought that many small changes could cause two lines of life to diverge. Whenever animals and plants find their way to a new home, for instance, they suffer, like émigrés in new countries. Some individuals fail, others adapt and prosper. As the more successful individuals reproduce, Darwin maintained, the new populations begins to differ from the ancestral one. If the two populations diverge widely enough, they become separate species. Change on that scale is known as macroevolution.
In Origin, Darwin estimates that a new species might take between ten thousand and fourteen thousand generations to arise. Until recently, most biologists assumed it would take at least that many, or maybe even millions of generations, before microevolutionary changes led to the origin of new species. So they assumed they could watch evolution by natural selection, but not the divergence of one species into separate, reproductively isolated species. Now that view is changing too.
Sockeye Salmon
 Not long ago, a young evolution-watcher named Andrew Hendry, a biologist at McGill University in Montreal, reported the results of a striking study of sockeye salmon. Sockeye tend to reproduce either in streams or along lake beaches. When the glaciers of the last ice age melted and retreated, about ten thousand years ago, they left behind thousands of new lakes. Salmon from streams swam into the lakes and stayed. Today their descendents tend to breed among themselves rather than with sockeyes that live in the streams. The fish in the lakes and streams are reproductively isolated from each other. So how fast did that happen?
Not long ago, a young evolution-watcher named Andrew Hendry, a biologist at McGill University in Montreal, reported the results of a striking study of sockeye salmon. Sockeye tend to reproduce either in streams or along lake beaches. When the glaciers of the last ice age melted and retreated, about ten thousand years ago, they left behind thousands of new lakes. Salmon from streams swam into the lakes and stayed. Today their descendents tend to breed among themselves rather than with sockeyes that live in the streams. The fish in the lakes and streams are reproductively isolated from each other. So how fast did that happen?
In the 1930s and 1940s, sockeye salmon were introduced into Lake Washington, in Washington State. Hundreds of thousands of their descendants now live and breed in Cedar River, which feeds the lake. By 1957 some of the introduced sockeye also colonized a beach along the lake called Pleasure Point, about four miles from the mouth of the Cedar River.
Hendry could tell whether a full-grown, breeding salmon had been born in the river or at the beach by examining the rings of its otoliths, or ear stones. Otolith rings reflect variations in water temperature while a fish embryo is developing. Water temperatures at the beach are relatively constant compared with the river temperatures. Hendry and his colleagues checked the otoliths and collected DNA samples from the fish and found that more than a third of the sockeye breeding at Pleasure Point had grown up in the river. They were immigrants.
With such a large number of immigrants, the two populations at Pleasure Point should have blended back together. But they hadn’t. So at breeding time many of the river sockeye that swam over to the beach must have been relatively unsuccessful at passing on their genes.
Hendry could also tell the stream fish and the beach fish apart just by looking at them. Where the sockeye’s breeding waters are swift-flowing, such as in the Cedar River, the males tend to be slender. Their courtship ritual and competition with other males requires them to turn sideways in strong current—an awkward maneuver for a male with a deep, roundish body. So in strong current, slender males have the better chance of passing on their genes. But in still waters, males with the deepest bodies have the best chance of getting mates. So beach males tend to be rounder-their dimensions greater from the top of the back to the bottom of the belly-than river males.
What about females? In the river, where currents and floods are forever shifting and swirling the gravel, females have to dig deep nests for their eggs. So the females in the river tend to be bigger than their lake-dwelling counterparts, because bigger females can dig deeper nests. Where the water is calmer, the gravel stays put, and shallower nests will do.
So all of the beachgoers, male and female, have adapted to life at Pleasure Point. Their adaptations are strong enough that reproductive isolation has evolved. How long did the evolution take? Hendry began studying the salmon’s reproductive isolation in 1992. At that time, the sockeyes in the stream and the ones at Pleasure Point had been breeding in their respective habitats for at most thirteen generations. That is so fast that, as Hendry and his colleagues point out, it may be possible someday soon to catch the next step, the origin of a new species.
Sticklebacks
 And it’s not just the sockeye salmon. Consider the three-spined stickleback. After the glaciers melted at the end of the last ice age, many sticklebacks swam out of the sea and into new glacial lakes—just at the salmon did. In the sea, sticklebacks wear heavy, bony body armor. In a lake they wear light armor. In a certain new pond in Bergen, Norway, during the past century, sticklebacks evolved toward the lighter armor in just thirty-one years. In Loberg Lake, Alaska, the same kind of change took only a dozen years. A generation for sticklebacks is two years. So that dramatic evolution took just six generations.
And it’s not just the sockeye salmon. Consider the three-spined stickleback. After the glaciers melted at the end of the last ice age, many sticklebacks swam out of the sea and into new glacial lakes—just at the salmon did. In the sea, sticklebacks wear heavy, bony body armor. In a lake they wear light armor. In a certain new pond in Bergen, Norway, during the past century, sticklebacks evolved toward the lighter armor in just thirty-one years. In Loberg Lake, Alaska, the same kind of change took only a dozen years. A generation for sticklebacks is two years. So that dramatic evolution took just six generations.
Dolph Sehluter, a former finch-watcher from the Galapagos and currently a biologist at the University of British Columbia in Vancouver, has shown that, along with the evolution of new body types, sticklebacks also evolve a taste for mates with the new traits. In other words, the adaptive push of sexual selection is going hand-in-hand with natural selection. Schluter has built experimental ponds in Vancouver to observe the phenomenon under controlled conditions, and the same patterns he found in isolated lakes repeat themselves in his ponds. So adaptation can sometimes drive sexual selection and accelerate reproductive isolation.
There are other developments in the evolution watch, too may to mention in this small space. Some of the fastest action is microscopic. Richard Lenski, a biologist at Michigan State University in East Lansing, watches the evolution of Escherichia coli. Because one generation takes only twenty minutes, and billions of E. coli can fit in a petri dish, the bacteria make ideal subjects for experimental evolution. Throw some E. coli into a new dish, for instance, with food they haven’t encountered before, and they will evolve and adapt—quickly at first and then more slowly, as they refine their fit with their new environment.
And then there are controversies. Science progresses and evolves by controversy, by internal debate and revision. In the United States these days one almost hates to mention that there are arguments among evolutionists. So often, they are taken out of context and hyperamplified to suggest that nothing about Darwinism is solid-that Darwin is dead. But research is messy because nature is messy, and fieldwork is some of the messiest research of all. It is precisely here at its jagged cutting edge that Darwinism is most vigorously alive.
The Peppered Moth

Not long ago, one of the most famous icons of the evolution watch toppled over: the story of the peppered moths, familiar to anyone who remembers biology 101. About half a century ago, the British evolutionist Bernard Kettlewell noted that certain moths in the British Isles had evolved into darker forms when the trunks of trees darkened with industrial pollution. When the trees lightened again, after clean air acts were passed, the moths had evolved into light forms again. Kettlewell claimed that dark moths resting on dark tree trunks were harder for birds to see; in each decade, moths of the right color were safer.
But in the past few years, workers have shown that Kettlewell’s explanation was too simplistic. For one thing, the moths don’t normally rest on tree trunks. In forty years of observation, only twice have moths been seen resting there. Nobody knows where they do rest. The moths did evolve rapidly, but no one can be certain why.
To me what remains most interesting is the light that studies such as Hendry’s, or the Grants’, may throw on the origin of species. It’s extraordinary that scientists are now examining the very beginnings of the process, at the level of beaks and fins, at the level of the genes. The explosion of evolution-watchers is a remarkable development in Darwin’s science. Even as the popular debate about evolution in America is reaching its most heated moment since the trial of John Scopes, evolutionary biologists are pursuing one of the most significant and surprising voyages of discovery since the young Darwin sailed into the Galapagos Archipelago aboard Her Majesty’s ship Beagle.
Not long ago I asked Hendry if his studies have changed the way he thinks about the origin of species. “Yes,” he replied without hesitation, “I think it’s occurring all over the place.”
